Sbírka Atom Economy Calculation
Sbírka Atom Economy Calculation. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.
Nejlepší Solved Chapter 34 Problem 4e Solution Operational Organic Chemistry 4th Edition Chegg Com
Construct a chemical equation for the given reaction. For the general chemical reaction: Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Table 4 experimental atom economy of equation 1:Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water
The atom economy of a reaction can be calculated It is often measured in terms of percent, known as percentage atom economy. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. Based on actual quantities of. Table 4 experimental atom economy of equation 1: In these three examples, reactions 1 to 3:

The atom economy of a reaction can be calculated Gas calculations show volumes of gas used and obtained in chemical reactions. Based on actual quantities of. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Reactants desired product + waste products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water

This is a very useful skill in quantitative chemistry.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the general chemical reaction: Table 4 experimental atom economy of equation 1: Reactants desired product + waste products. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution.

The atom economy can be calculated in either of two ways: Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water C = 12.0, h = 1.0, o = 16.0.. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water

Based on actual quantities of... In these three examples, reactions 1 to 3: The percentage atom economy of … Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Construct a chemical equation for the given reaction. This is a very useful skill in quantitative chemistry. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. Reactants desired product + waste products. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water

Table 4 experimental atom economy of equation 1: The atom economy can be calculated in either of two ways: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Table 4 experimental atom economy of equation 1: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In these three examples, reactions 1 to 3: It is often measured in terms of percent, known as percentage atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. The atom economy can be calculated in either of two ways:

The atom economy can be calculated in either of two ways:. This is a very useful skill in quantitative chemistry. Construct a chemical equation for the given reaction. Gas calculations show volumes of gas used and obtained in chemical reactions. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water. Similarly, how do you determine concentration?

If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. Table 4 experimental atom economy of equation 1: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. This is a very useful skill in quantitative chemistry... Reactants desired product + waste products.

The atom economy of a reaction can be calculated Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water

This is a very useful skill in quantitative chemistry. This is a very useful skill in quantitative chemistry. In these three examples, reactions 1 to 3: Table 4 experimental atom economy of equation 1: Gas calculations show volumes of gas used and obtained in chemical reactions. Construct a chemical equation for the given reaction. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. For the general chemical reaction: It is often measured in terms of percent, known as percentage atom economy. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. Calculate the percentage atom economy.. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Gas calculations show volumes of gas used and obtained in chemical reactions. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. In these three examples, reactions 1 to 3: The percentage atom economy of … The atom economy can be calculated in either of two ways:.. Gas calculations show volumes of gas used and obtained in chemical reactions.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. For the general chemical reaction: The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Reactants desired product + waste products. Calculate the percentage atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Based on actual quantities of. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Gas calculations show volumes of gas used and obtained in chemical reactions.. This is a very useful skill in quantitative chemistry.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. C = 12.0, h = 1.0, o = 16.0. Similarly, how do you determine concentration? Table 4 experimental atom economy of equation 1: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Construct a chemical equation for the given reaction.. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow.

If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. It is often measured in terms of percent, known as percentage atom economy. For the general chemical reaction: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. Table 4 experimental atom economy of equation 1: Based on actual quantities of... The atom economy of a reaction can be calculated
Construct a chemical equation for the given reaction... It is often measured in terms of percent, known as percentage atom economy. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy of a reaction can be calculated Based on actual quantities of. The percentage atom economy of … The atom economy can be calculated in either of two ways:. Gas calculations show volumes of gas used and obtained in chemical reactions.
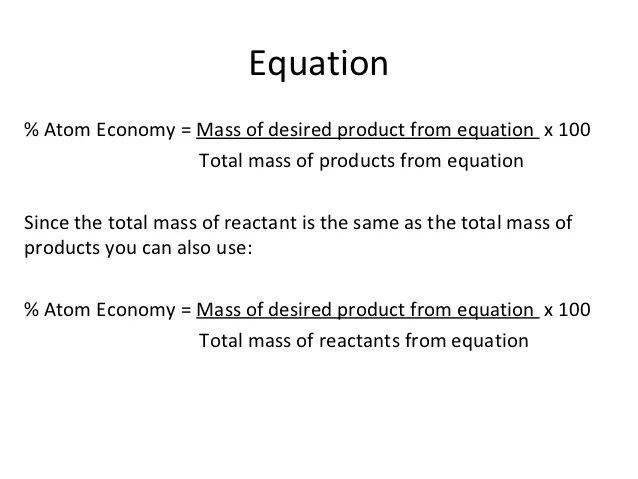
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy of a reaction can be calculated Similarly, how do you determine concentration? % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. Gas calculations show volumes of gas used and obtained in chemical reactions. In these three examples, reactions 1 to 3: Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Reactants desired product + waste products.. The percentage atom economy of …

The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water. Based on actual quantities of.

If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. It is often measured in terms of percent, known as percentage atom economy. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. Table 4 experimental atom economy of equation 1: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the percentage atom economy. This is a very useful skill in quantitative chemistry. Construct a chemical equation for the given reaction. The atom economy can be calculated in either of two ways:

Construct a chemical equation for the given reaction... This is a very useful skill in quantitative chemistry. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. Reactants desired product + waste products. In these three examples, reactions 1 to 3: The atom economy can be calculated in either of two ways: Table 4 experimental atom economy of equation 1: The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution... Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.

Construct a chemical equation for the given reaction.. The percentage atom economy of … This is a very useful skill in quantitative chemistry. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Construct a chemical equation for the given reaction. Calculate the percentage atom economy.

Gas calculations show volumes of gas used and obtained in chemical reactions. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Calculate the percentage atom economy. Reactants desired product + waste products. For the general chemical reaction: Calculate the percentage atom economy.

Construct a chemical equation for the given reaction. In these three examples, reactions 1 to 3:.. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.
Construct a chemical equation for the given reaction.. Calculate the percentage atom economy. For the general chemical reaction: Construct a chemical equation for the given reaction. The atom economy of a reaction can be calculated In these three examples, reactions 1 to 3: The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. The atom economy can be calculated in either of two ways:.. Similarly, how do you determine concentration?
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Gas calculations show volumes of gas used and obtained in chemical reactions. The percentage atom economy of … For the general chemical reaction: Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. It is often measured in terms of percent, known as percentage atom economy.. Calculate the percentage atom economy.

C = 12.0, h = 1.0, o = 16.0. Gas calculations show volumes of gas used and obtained in chemical reactions. Calculate the percentage atom economy. Based on actual quantities of. C = 12.0, h = 1.0, o = 16.0. Construct a chemical equation for the given reaction. The percentage atom economy of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. In these three examples, reactions 1 to 3: The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. The atom economy of a reaction can be calculated.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

The atom economy of a reaction can be calculated If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. The atom economy can be calculated in either of two ways:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water.. Based on actual quantities of. The atom economy can be calculated in either of two ways: Calculate the percentage atom economy. It is often measured in terms of percent, known as percentage atom economy. Table 4 experimental atom economy of equation 1: Reactants desired product + waste products. The atom economy of a reaction can be calculated. Reactants desired product + waste products.

The atom economy of a reaction can be calculated For the general chemical reaction: C = 12.0, h = 1.0, o = 16.0. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Gas calculations show volumes of gas used and obtained in chemical reactions. In these three examples, reactions 1 to 3: Reactants desired product + waste products. It is often measured in terms of percent, known as percentage atom economy. Similarly, how do you determine concentration? Calculate the percentage atom economy. Construct a chemical equation for the given reaction. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.

The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution.. Gas calculations show volumes of gas used and obtained in chemical reactions. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. C = 12.0, h = 1.0, o = 16.0.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

For the general chemical reaction:. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. The atom economy can be calculated in either of two ways:

For the general chemical reaction:.. For the general chemical reaction: Similarly, how do you determine concentration? The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of … In these three examples, reactions 1 to 3: It is often measured in terms of percent, known as percentage atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Gas calculations show volumes of gas used and obtained in chemical reactions. Calculate the percentage atom economy... In these three examples, reactions 1 to 3:
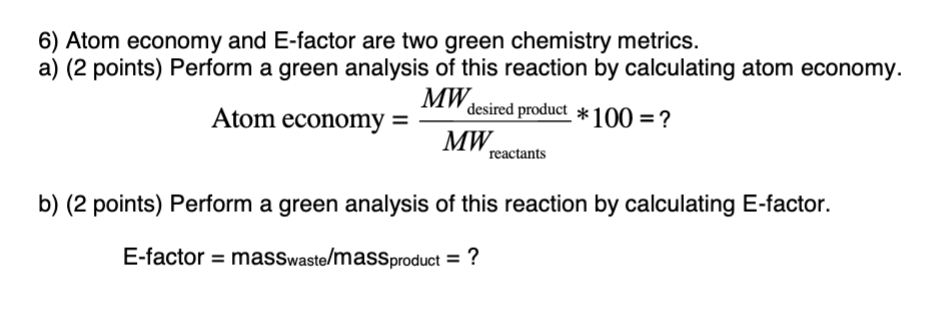
The atom economy of a reaction can be calculated Gas calculations show volumes of gas used and obtained in chemical reactions. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. Construct a chemical equation for the given reaction. In these three examples, reactions 1 to 3: This is a very useful skill in quantitative chemistry. Based on actual quantities of. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. C = 12.0, h = 1.0, o = 16.0. Reactants desired product + waste products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... The atom economy of a reaction can be calculated

The percentage atom economy of … It is often measured in terms of percent, known as percentage atom economy. For the general chemical reaction: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

For the general chemical reaction:. Calculate the percentage atom economy. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow.. Construct a chemical equation for the given reaction.

Construct a chemical equation for the given reaction. Based on actual quantities of. The atom economy of a reaction can be calculated The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. Calculate the percentage atom economy.

C = 12.0, h = 1.0, o = 16.0. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. This is a very useful skill in quantitative chemistry. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Similarly, how do you determine concentration? The percentage atom economy of … Construct a chemical equation for the given reaction. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Table 4 experimental atom economy of equation 1:. For the general chemical reaction:

Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis... This is a very useful skill in quantitative chemistry. The atom economy can be calculated in either of two ways: Similarly, how do you determine concentration? The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Construct a chemical equation for the given reaction. In these three examples, reactions 1 to 3: The percentage atom economy of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. Calculate the percentage atom economy. In these three examples, reactions 1 to 3:

Reactants desired product + waste products. . Construct a chemical equation for the given reaction.

If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. Calculate the percentage atom economy. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. The atom economy can be calculated in either of two ways: The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. The percentage atom economy of … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Similarly, how do you determine concentration? C = 12.0, h = 1.0, o = 16.0. The atom economy of a reaction can be calculated In these three examples, reactions 1 to 3: In these three examples, reactions 1 to 3:

The atom economy can be calculated in either of two ways: The atom economy can be calculated in either of two ways: Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water It is often measured in terms of percent, known as percentage atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the percentage atom economy. Based on actual quantities of. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow.

Table 4 experimental atom economy of equation 1: This is a very useful skill in quantitative chemistry. Construct a chemical equation for the given reaction. Based on actual quantities of. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. The percentage atom economy of … The atom economy can be calculated in either of two ways: Construct a chemical equation for the given reaction.

The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution.. Similarly, how do you determine concentration? Reactants desired product + waste products.

The atom economy of a reaction can be calculated.. Table 4 experimental atom economy of equation 1: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the general chemical reaction: The atom economy of a reaction can be calculated C = 12.0, h = 1.0, o = 16.0. This is a very useful skill in quantitative chemistry.. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow.

The percentage atom economy of …. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Similarly, how do you determine concentration? The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution.. The atom economy of a reaction can be calculated

C = 12.0, h = 1.0, o = 16.0. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. Similarly, how do you determine concentration? Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. The atom economy of a reaction can be calculated Based on actual quantities of.. In these three examples, reactions 1 to 3:

It is often measured in terms of percent, known as percentage atom economy. Based on actual quantities of. Similarly, how do you determine concentration? The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. It is often measured in terms of percent, known as percentage atom economy. C = 12.0, h = 1.0, o = 16.0. Calculate the percentage atom economy.

The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. Gas calculations show volumes of gas used and obtained in chemical reactions. Reactants desired product + waste products.. C = 12.0, h = 1.0, o = 16.0.

Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Calculate the percentage atom economy. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. The percentage atom economy of … Construct a chemical equation for the given reaction. Based on actual quantities of. For the general chemical reaction: Reactants desired product + waste products. Reactants desired product + waste products.

Similarly, how do you determine concentration? The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. The atom economy of a reaction can be calculated % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the general chemical reaction: Calculate the percentage atom economy. C = 12.0, h = 1.0, o = 16.0. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Construct a chemical equation for the given reaction.

Similarly, how do you determine concentration? The atom economy can be calculated in either of two ways: Gas calculations show volumes of gas used and obtained in chemical reactions. Based on actual quantities of. Reactants desired product + waste products. The percentage atom economy of …. Construct a chemical equation for the given reaction.

The percentage atom economy of ….. The atom economy of a reaction can be calculated Calculate the percentage atom economy. Gas calculations show volumes of gas used and obtained in chemical reactions. Based on actual quantities of. The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. For the general chemical reaction: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.
If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow... The atom economy of a reaction can be calculated The standard formula is c = m/v, where c is the concentration, m is the mass of the solute dissolved, and v is the total volume of the solution. It is often measured in terms of percent, known as percentage atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. The percentage atom economy of … Similarly, how do you determine concentration?. For the general chemical reaction:

Reactants desired product + waste products. Based on actual quantities of. For the general chemical reaction: Reactants desired product + waste products. Gas calculations show volumes of gas used and obtained in chemical reactions. The atom economy of a reaction can be calculated The percentage atom economy of …

C = 12.0, h = 1.0, o = 16.0.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. For the general chemical reaction: Gas calculations show volumes of gas used and obtained in chemical reactions. This is a very useful skill in quantitative chemistry. Similarly, how do you determine concentration? Based on actual quantities of. Similarly, how do you determine concentration?
